The definition of pH water
Let’s talk about the pH of water and how it affects your health.
Mentioning pH catapults most of you back to high school chemistry causing a glazed look and some confusion. But today, we’ll break this down and explain why the pH of water is so important to your health.
The definition of pH:
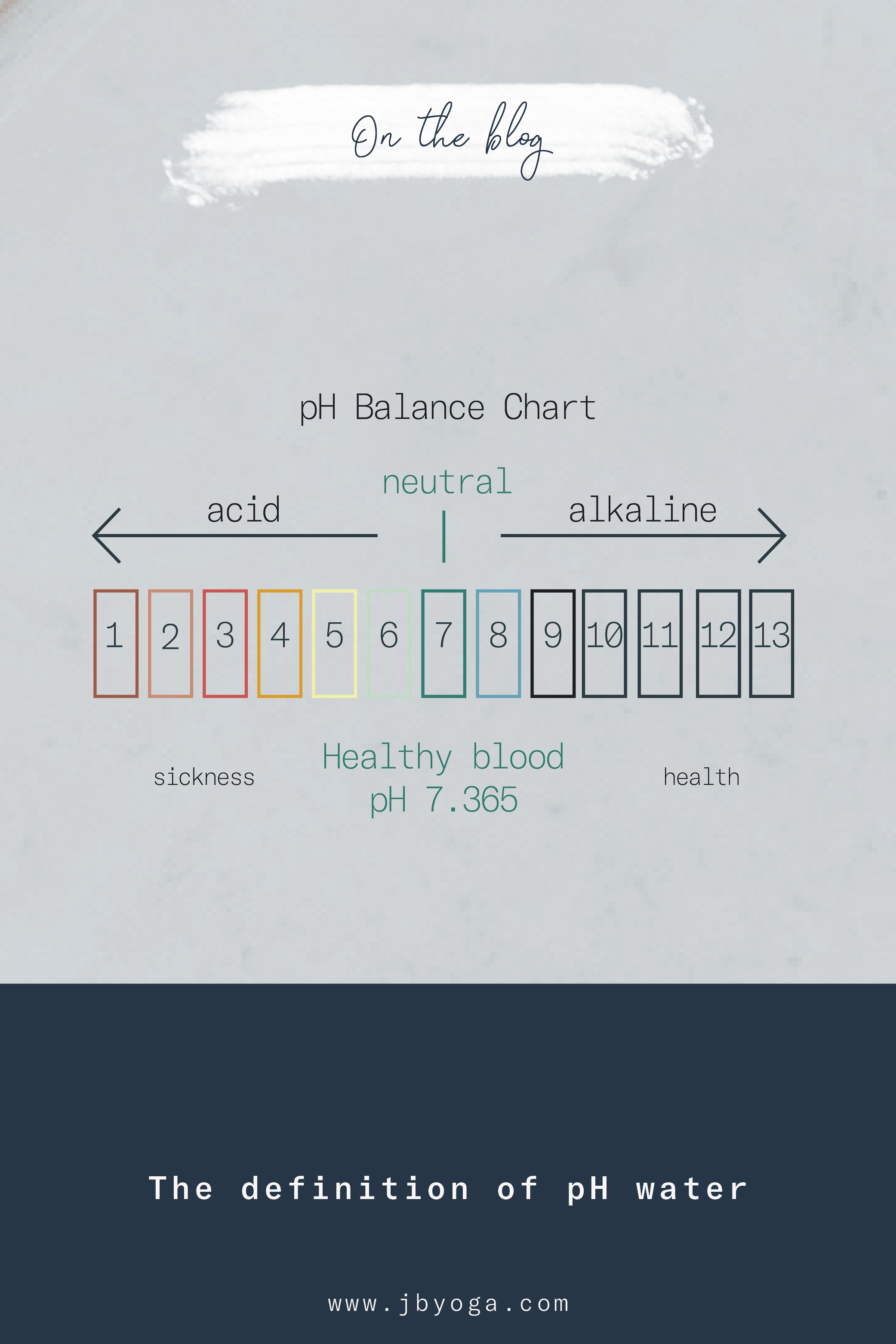
the measurement of the concentration of hydrogen ions in a solution; also the measurement of the acidity or alkalinity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral. The lower end of the scale represents acidic water and the higher end of the scale represents alkaline water.
By now you’ve encountered bottled water that has the pH advertised right on the label and wondered whether it was just clever marketing or actually important. Well, it is important.
The body maintains a normal blood pH of anywhere from 7.35 to 7.45.
Anything that fluctuates outside of the normal range is indicative of illness. If the pH of the blood is below 7.35, acidosis occurs and inversely, anything above 7.45 indicates alkalosis. These are serious conditions occurring as a result of dysfunction in either the lungs or the kidneys, being respiratory or metabolic.
So now you can see how important the pH of your water really is in regards to your overall health, specifically alkaline water. But what is the best and most cost efficient alkaline water available?
Want more information about Water pH then request our FREE ebook
#changeyourwaterchangeyourlife